| Symbol | Designation Number | Title | Definition per BS EN ISO 15223-1:2021 | |
 |
5.1.1 | Manufacturer Symbol | Indicates the official manufacturer on record for the device. Will be accompanied by the name and address of the manufacturer. | |
 |
EN ISO 15223-1 | Caution Symbol | Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or that the current situation needs operator awareness or operator action in order to avoid undesirable consequences. | |
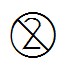 |
5.4.2 | Do not re-use | Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure. | |
 |
5.1.6 | Catalog number | Indicates the manufacturer’s catalog number so that the medical device can be identified. | |
 |
5.1.5 | Batch code | Indicates the manufacturer’s batch code so that the batch or lot can be identified. | |
| Model Number | This symbol shall be accompanied by the model number or the part number adjacent to the symbol | |||
 |
5.3.2 | Sterilized using ethylene oxide | Indicates a medical device that has been sterilized using ethylene oxide. | |
 |
5.2.3 | Sterilized using irradiation. | Indicates a medical device that has been sterilized using irradiation. | |
 |
5.1.4 | Use-by date | Indicates the date after which the medical device is not to be used. | |
 |
5.4.3 | Consult instructions for use |
Indicates the need for the user to consult the instructions for use. | |
 |
EN ISO 15223-1 | Keep Dry | Keep Dry | |
 |
5.3.2 | Keep away from sunlight | Indicates a medical device that needs protection from light sources. | |
 |
EN ISO 15223-1 | Keep away from heat and radioactive sources. | Indicates that a device needs to be kept away from light sources; this symbol can also mean “keep away from sunlight and radioactive sources”. Radioactive sources include ionizing radiation | |
| 5.3.7 | Temperature limit | Indicates the temperature limits to which the medical device can be safely exposed. | ||
| EN ISO 15223-1 | Lower Limit of Temperature | Lower limit of temperature | ||
| EN ISO 15223-1 | Upper Limit of Temperature | Upper limit of temperature | ||
 |
5.2.7 | Non-sterile | Indicates a medical device that has not been subjected to a sterilization process. | |
 |
5.2.6 | Do not resterilize | Indicates a medical device that is not to be resterilized. | |
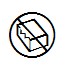 |
5.2.8 | Do not use if package is damaged |
Indicates a medical device that should not be used if the package has been damaged or opened. | |
| Symbol | Reference | Title | Definition Per Referenced Regulation | |
| 21 CFR 801.109 | RX Only | Caution: Federal law restricts this device to sale by or on the order of a physician or a properly licensed practitioner. | ||
 |
ASTM F2502-23 | MR Safe | Indicated an item poses no known hazards relating from exposure to any MR environment. | |
 |
ASTM F2502-23 | MR Conditional | Indicates an item that has demonstrated safety in the MR environment within defined conditions. | |
 |
BS EN 62570
ASTM F2502-23 |
MR Unsafe | Indicates an item which poses unacceptable risks to the patient, medical staff or other persons within the MR environment. | |
 |
EN ISO 15223-1 | Country of Manufacture | Indicates the Country of Origin for manufacturing of the products. "CC" replaced by applicable two letter country code defined in ISO 3166-1. | |
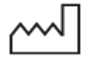 |
EN ISO 15223-1 | Date of Manufacture | Indicates the date when the medical device was manufactured. | |
| EN ISO 15223-1 | Translation symbol | This symbol shall be accompanied by the name and address of the entity that is responsible for the translation activity adjacent to the symbol. Only to be used when the translation is performed by someone other than the manufacturer. | ||
 |
EN ISO 15223-1 | Repackaging Symbol | This symbol shall be accompanied by the name and address of the entity that is responsible for the repackaging activity adjacent to the symbol. Only to be used when the activity is performed by someone other than the manufacturer. | |
| EN ISO 15223-1 | UDI Symbol | Optional symbol may be used if there are multiple data carriers or barcodes on the label. | ||
 |
EN ISO 15223-1 | Medical Device | Indicates the item is a medical device. | |
 |
ICU Medical | Quantity | Indicates the # of unit per package. | |
 |
EN ISO 15223-1 | Importer | This symbol shall be accompanied by the name and address of the importing entity adjacent to the symbol. | |
 |
EN ISO 15223-1 | Distributor | This symbol shall be accompanied by the name and address of the distributing entity adjacent to the symbol. | |
| EN ISO 15223-1 | Single Sterile Barrier System | Indicates a single sterile barrier system. | ||
| EN ISO 15223-1 | Double Sterile Barrier System | Indicates two sterile barrier systems. | ||
| EN ISO 15223-1 | Single Sterile Barrier System with protective packaging inside | Indicates a single sterile barrier system with protective packaging inside. | ||
| EN ISO 15223-1 | Single Sterile Barrier System with protective packaging outside | Indicates a single sterile barrier system with protective packaging outside. | ||
 |
93/42/EEC | CE Mark Including SGS Identification Number | Indicates the product conforms with the essential requirements in the European Medical Devices Directive 93/42/EEC. | |
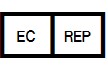 |
5.1.2 | Authorized representative in the European Community | Indicates the Authorized representative in the European Community. | |
 |
EN ISO 15223-1 | Combined Symbol | Sterilization symbols may be combined to indicate sterilization method and barrier | |
 |
EN ISO 15223-1 | Combined Symbol | Sterilization symbols may be combined to indicate sterilization method and barrier |
